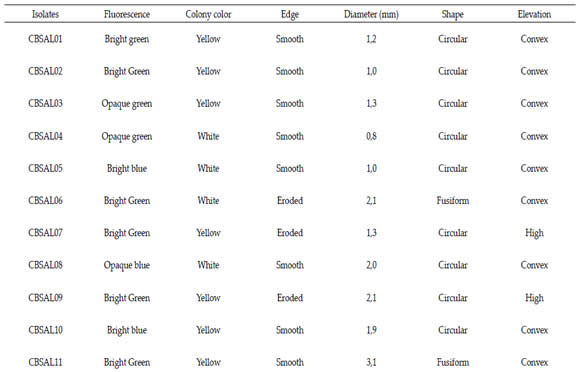
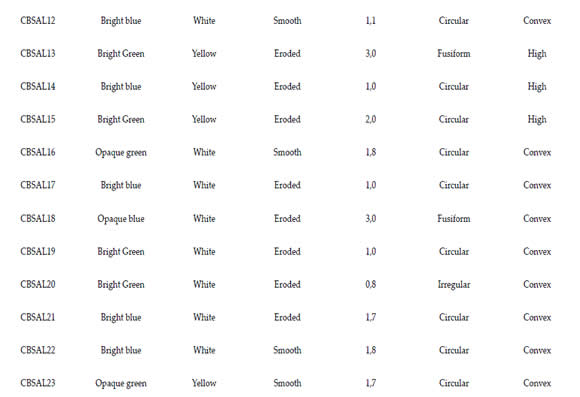
Tabla 1. Perfil morfológico de aislados de Pseudomonas fluorescente de ajo
BIOLOGÍA DEL SUELO
Plant growth promoting bacteria from garlic sowed at curitibanos micro-Region - Santa Catarina - Brazil
Bacterias promotoras de crescimento de ajo cultivado en la microrregión de curitibanos - Santa Catarina - Brasil
Gloria R. Botelho*1; Mariane R. Leoncio; Bruna Orsi; Elisa Coser; Géssica R. Eutrópio; Rafael D. De Armas; Cláudio R. F. Sousa Soares; Jerri E. Zilli
1 Universidade Federal de Santa Catarina (UFSC) campus de Curitibanos - Centro de Ciências Rurais - Brasil
* Autor de contacto: gloria.botelho@ufsc.br
Recibido: 18/1/2019
Recibido con revisiones: 19/4/2019
Aceptado: 22/4/2019
ABSTRACT
Garlic has great economic and social importance for the region of Curitibanos (SC). However, a number of factors
has hindered production. One of these factors is the high cost of fertilizers, especially the nitrogenous ones, an
element of which the crop is highly demanding. To reduce the impacts, the use of PGPR (Plant Growth Promoting
Rhizobacteria), especially fluorescent Pseudomonas to induce plant development and their production has been
widely studied. However, little is known about these microorganisms and garlic. Fluorescent Pseudomonas were
isolated from rhizosphere of garlic cultivated in soil of the Curitibanos region (SC). Twenty-three isolates were
phenotypical and genotypically analyzed. The phosphate solubiliziation, IAA (Indole Acetic Acid) production and
Biological Nitrogen Fixation (BNF) in vitro, besides the effect on plant under controlled conditions were evaluated.
The phenotypic characteristics matched to those described for the group of fluorescent Pseudomonas. Fifty
two percent of isolates solubilized Ca3(PO4)2, 69% presented FBN and 72% produced IAA. Genetically, all the
isolates belonged to the genus Pseudomonas and resembled to Pseudomonas kribbensis, P. azotoformans or
P. baetica. The strain CBSAL02 induced significant increase at fourth leaf size, having high correlation to the
bulbification, suggesting its potential as a growth promoter and, consequently, production.
Key words: Rhizobacteria, Alliaceae, Fluorescent Pseudomonas.
RESUMEN
El ajo tiene una gran importancia económica y social para la región de Curitibanos (SC). Sin embargo, una serie
de factores ha impedido la producción. Uno de estos factores es el alto costo de los fertilizantes, especialmente
los nitrogenados, un elemento por el cual el cultivo es muy exigente. Para reducir los impactos, se ha estudiado
ampliamente el uso de RPCP (Rizobacterias promotoras del crecimiento de las plantas), especialmente Pseudomonas
fluorescentes para inducir el desarrollo de las plantas y su producción. Sin embargo, poco se sabe sobre
estos microorganismos y el ajo. Las Pseudomonas fluorescentes se aislaron de la rizosfera de ajo cultivado en el
suelo de la región de Curitibanos (SC). Veintitrés aislados fueron fenotípicos y genotípicamente analizados. Se
evaluaron la solubilización de fosfato, la producción de AAI (ácido acético indol) y la fijación biológica de nitrógeno
(FBN) in vitro, además del efecto en la planta bajo condiciones controladas. Las características fenotípicas
coinciden con las descritas para el grupo de Pseudomonas fluorescentes. Cincuenta y dos por ciento de los aislados
solubilizaron Ca3(PO4)2, 69% presentaron FBN y 72% produjeron AAI. Genéticamente, todos los aislados
pertenecían al género Pseudomonas y se parecían a Pseudomonas kribbensis, P. azotoformans o P. baetica. La
cepa CBSAL02 indujo un aumento significativo en el tamaño de la cuarta hoja, teniendo una alta correlación con
la bulbificación, lo que sugiere su potencial como promotor del crecimiento y, en consecuencia, la producción.
Palabras clave: Rizobacterias, Alliaceae, Pseudomonas fluorescente.
INTRODUCTION
Garlic (Allium sativum) is the fourth vegetable of economic relevance of Brazil. It is estimated that the cultivated area in the country at 2016/2017 harvest was 11,156 hectares and the production of 132,870 tons (Conab, 2017). Currently, Brazil is the second consumer and the world largest importer of garlic (Souza & Macedo, 2009). Socially, it is considered one of the most important vegetables in the country, because small farmers do much of the farming.
In Santa Catarina, garlic culture is among the four main vegetables grown in the state, alongside onions, potatoes and tomatoes (Biasi, 2006). Initially, garlic culture was introduced in the Catarinense plateau, which displays environmental conditions for its development, such as low temperatures to the dormancy breakdown. For several years, this region was considered the garlic capital (Lucini, 2009).
However, economic and phytosanitary factors have led to decrease garlic production in the state of Santa Catarina. Currently, due to the importance to the state's economy, alternatives are sought to increase its production, through alternatives practices that promote the reduction of its costs. Among the garlic production-related costs, the agricultural inputs, such as fertilizers and agrochemicals, are the highest. These also have a high degree of impact on the environment. Reducing their use is therefore an economic and environmental demand, since it aims at the sustainability of agricultural systems.
In this context, the study of some microorganisms in the soil that are essential to plants, because they can increase the supply of nutrients, such as nitrogen and phosphorus (Melo & Valadrini, 1995), has been widely studied (Moreira et al. 2010; Hungria & Mendes, 2015). Among these microorganisms, there are the Plant Growth Promoting Rhizobacteria Plant Growth (PGPR), which are bacteria that inhabit the rhizosphere and promote a variety of benefits for the growth of plant species (Botelho & Mendonça-Hagler, 2006; Drogue et al., 2012; Sivasakthi et al., 2014; Gouda et al., 2018).
PGPR can act directly in promoting growth or indirectly, for their contribution in the biological control of plant pathogens (Voisard et al., 1994; Freitas & Aguilar-Vildoso, 2004; Turatto et al., 2018). Among the most studied and promising PGPR are fluorescent Pseudomonas (Novakowiski et al., 2011; Ogata-Gutiérrez et al., 2017).
Pseudomonas genus stand out for developing in varied environments and substrates, due to its metabolic diversity. So, they are found in water and soil, especially in the rhizosphere (Botelho & Mendonça-Hagler, 2006; Ferreira et al., 2009). The fluorescent Pseudomonas are Gram-negative bacteria and produce yellow-green fluorescent pigment on King B medium when observed at wavelengths in the ultraviolet range. The most important species of this group are P. fluorescens and P. putida (Coelho, 2006).
There are several direct effect mechanisms in plant development promotion by Pseudomonas. Among these, mention may be made of solubilization and increased input of nutrients such as P, production of plant growth regulators (Botelho & Mendonça-Hagler, 2006; Agaras et al., 2015). As an indirect effect, they can suppress phytopathogens, through mechanisms such as siderophore production and antibiotics (Loper & Buyer, 1991; Voisard et al., 1994; Raaijmakers & Mazzola, 2012; Yu et al. 2018).
However, studies on the effects of these bacteria on garlic crop are still scarce. As these rhizobacteria have great potential for agricultural use, reducing economic and environmental costs, the goal was to isolate and characterize PGPR, especially fluorescent Pseudomonas, as well as to check their growth promotion potential for garlic.
MATERIALS AND METHODS
Soil of the micro-region of Curitibanos (SC) was collected from the farm Dias, at district of Horizolandia that were sampled from cultivated area. Samples were taken in August 2013, during the winter, from rhizosphere garlic soil at 15cm of depth. It was classified as Cambisols associated to Nitisols (Dos Santos et al., 2013). In the region, it grows garlic during winter and corn and beans during spring/summer. That soil was used for the rhizobacteria isolation from garlic roots in greenhouse.
Pseudomonas ISOLATION
For the isolation of bacteria from the garlic rhizosphere, two pots containing 5L of soil from cultivated area received four garlic bulbs. The pots were kept in greenhouse for 30 days, until rhizobacteria isolation. After this period, 10g of the soil around the root and 10g of roots were collected. Before processed, the roots were disinfected with alcohol 95% for 30s, 3% bleach solution for 10 minutes, then washed five times with sterile distilled water and then macerated. Both soil and roots were submitted to serial dilution. An aliquot of 0.1 mL of each dilution transferred into plates containing King B medium (King et al., 1954) for isolation of fluorescent Pseudomonas. After incubation, the isolates that showed fluorescence under ultraviolet light (365 nm) were purified by subcultures on King B medium and stored at -20 ° C in 40% glycerol cryotubes.
PHENOTYPIC CHARACTERIZATION
The isolates were characterized phenotypically by evaluating morphological and physiological activities. For morphological characterization the following properties were evaluated: (circular, spindle-shaped or irregular) size (<1 cm, 1 - 2 cm;> 2 cm), elevation (convex or high), edge (smooth or eroded) and color of the colony (yellow or white). There were evaluated characteristics related to the fluorescence, such as color (blue or green) and intensity (bright or opaque).
Biochemical characterization was determined by glucose fermentation, Vogues Proskauer, urease, catalase and growth at 41°C, according to Ribeiro & Soares (2002). Bacteria were grown in liquid King B medium for 24 hours at 30°C. An aliquot was transferred to the tubes containing 5mL of specific media for each test (glucose fermentation, Vogues Proskauer and urease) in triplicate for each isolate. The tubes were incubated at 30 ° C for 48 hours. For growth at 41 ° C was used 5mL of King B medium liquid.
EVALUATION OF PLANT GROWTH-INDUCING MECHANISMS
The growth promotion potential was assessed by determining the phosphate solubilizing capacity, production IAA (Indole Acetic Acid) and Biological Nitrogen Fixation (BNF).
For the evaluation of phosphate solubilizing capacity, a medium containing tribasic calcium phosphate was used (10 g L-1 glucose; 5 g L-1 de NH4Cl; 1 g L-1 de MgSO4.7H2O; 4g L-1 de Ca3PO4; 15g L-1 de agar; pH 6,5). The isolates were previously grown on King B medium liquid at 28°C for 24 hours. Then, 0,1mL were transferred to plates containing the media, establishing four isolates per plate arranged at equidistant points. For each group of four isolates, there were five replicates. Plates were incubated at 28°C and at every three days, colorless solubilization halos diameters around colonies were measured with a millimeter ruler, for nine days.
Isolates production of IAA was qualitatively evaluated through the colorimetric methodology adapted by Cattelan (1999). Isolates were previously inoculated in 5mL of King B liquid medium at a 28 °C, for 24 hours. Then, 0,1 mL of each suspension was placed on plates containing TSA (Tripticase Soy Agar) 1/10, plus 5 mM de Ltryptophan. Each plate received 12 isolates and each set had three replicates. Thereafter, previously sterilized nitrocellulose membranes were placed on each plate that were incubated at 28° C for 24 hours. After this period, the membranes were removed and saturated with Salkowski solution. The reddish halo in the membrane around the isolate's colonies indicated the production of IAA.
To assess the ability of BNF, the isolates were previously cultured in liquid LB medium for 24 h at 28°C. Then, 1 mL of each of the isolates was transferred to the center of vials containing semi-solid NFB medium modified by Döbereiner et al. (1995). These were incubated for 24 h at 28°C. Thereafter, it was observed isolates pellicle formation in the medium, as a positive reaction.
DNA EXTRACTION, PCR AMPLIFICATION AND SEQUENCING OF PCR FRAGMENTS
Genomic DNA of the strains was extracted using the kit Wizard DNA purification system (Promega). The 16S rRNA gene was amplified with the same primers and PCR conditions used before (Pires et al., 2018). The sequencing was performed in the, using the same primers used to the PCR for the both senses. Forward and reverse readings of the 16S rRNA were assembled using the Bionumerics softwre (version 7.0). Multiple sequence alignments were performed using Muscle through MEGA 7.0. Neighbor Joining phylogenetic reconstructions were completed using MEGA 7.0 for single gene sequences (16S rRNA). The distance matrices were calculated using the best fit substitution model Tamura 3-parameter estimated and the robustness of the tree nodes was evaluated with a bootstrap analysis using 1000 pseudoreplicates. The software default parameters were considered in all the analyses. Sequences of type strains used for alignment and phylogenetic analysis were retrieved from Gen- Bank (http://www.ncbi.nlm.nih.gov/genbank/).
NDUCTION ANALYSIS OF THE DEVELOPMENT OF GARLIC.
The evaluation of the growth promotion in the plant was carried out in a greenhouse, using four selected isolates, based on the results obtained from the assessments of phosphate solubilization capacity and IAA production.
Each bacterium was inoculated in erlenmeyer flasks containing 500 mL of liquid King B medium and incubated for 24 h at 28°C. The garlic bulbs were previously subjected to disinfestation in 70% ethanol, followed by immersion in 10% sodium hypochlorite for 3 minutes and five washes in sterile distilled water. Then, they were immersed in the bacterial suspension, where they remained for 40 minutes. After this, they were placed in sterile Petri dishes in a laminar flow to dryness. The number of CFUs in the bacterial suspensions was determined by serial dilution and counting on plates containing King B medium. In the control treatment, the procedure for the bulbils was similar to the others, however without inoculation.
Pots filled with 3L of vermiculite: sand (2:1) sterilized were sowed with two garlic bulbs (with or without inoculation). The nutrients supplied weekly out through nutrient solution based in Hoagland & Arnon (1950) and the sterile water each three days.
At the end of 110 days after sowing, plant development was evaluated by plant height, fourth leaf insertion and size and wet and dry weights of shoot and root. The experimental design was completely randomized, with five replicates and five treatments (four fluorescent Pseudomonas isolates and an uninoculated control). Data were evaluated by variance analysis and Tukey test comparison of means with 5% significance level. To validate the variance analysis the Bartlett test was used to verify homoscedasticity and the Shapiro-Wilks test, both with significance levels of 1% and 5%, respectively.
RESULTS
It was isolated 23 bacteria, from rhizospheric soil and from roots. All isolates were gram negative and showed fluorescence under ultraviolet light (365 nm), suggesting they belong to Pseudomonas fluorescent group (King et al.,1954).
By morphological analysis, most isolates showed green fluorescence (60,9%) and the intensity of the bright type (73,7%). The other characteristics that prevailed among the isolates were white color colonies (52,2%), size between 1,0- 2,0mm (69,6%), smooth edge (52,2%), circular (78,3%) and convex elevation (78,3%). Only five isolates had high colony. The biochemical evaluation results of fluorescent Pseudomonas are exposed at Table 2. All isolates were negative for glucose fermentation Vogues Proskauer test. Most isolates were able to degrade urea (82,6%). Only four were not able to degrade urea (CBSAL17, 18, 21, 23). Most of the isolates had a positive reaction for catalase production (82,6%). Only four isolates showed no reaction (CBSAL03, 14, 17, 23). All isolates showed no growth at 41°C. CBSAL17 and CBSAL23 isolates showed negative reaction for all the biochemical tests carried out.
Table 1. Morphological profile of garlic fluorescent Pseudomonas isolates.
Tabla 1. Perfil morfológico de aislados de Pseudomonas fluorescente de ajo


Table 2. Biochemical profile of garlic fluorescent Pseudomonas isolates.
Tabla 2. Perfil bioquímico de aislados de Pseudomonas fluorescente de ajo.
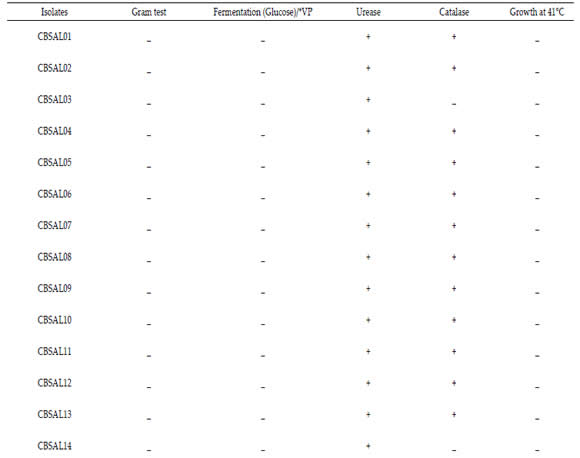
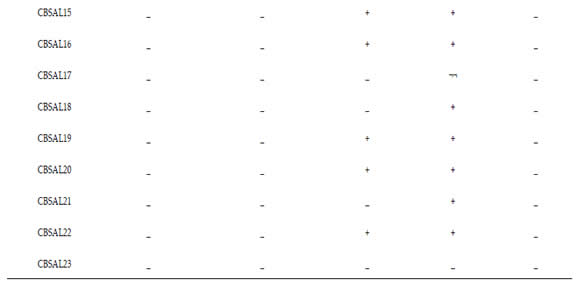
*V.P. - Vogues Proskauer; ( - ) - Negative; ( + ) – Positive.
GENOTYPIC CHARACTERIZATION AND ISOLATES IDENTIFICATION
The sequences of 16S rRNA obtained were initially submitted to similarity analysis in BLAST, choosing the option type material. All isolates presented as first hit strains belong from Pseudomonas (Figure 1). Strains CBSAL01, CBSAL13, CBSAL15, CBSAL10, CBSAL10, CBSAL09, CBSAL02, CBSAL02, CBSAL04, CBSAL06, CBSAL07, CBSAL07, CBSAL18, CBSAL19, CBSAL20, CBSAL10 and CBSAL03 showed 99% similarity to strain 46-2 of Pseudomonas kribbensis. On the other hand, strains CBSAL14, CBSAL21, CBSAL05, CBSAL17 and CBSAL16 showed 99% similarity with the strain LMG 21611 of P. azotoformans and, strains CBSAL23, CBSAL12 and CBSAL08 in the same way had high similarity with the strain a390 of P. baetica (Figure 1).
Subsequently, it was performed the phylogenetic analysis of the 16S rRNA sequences from the novel Pseudomonas isolates together with sequences from the three closely related types strains (retrieved from the genebank). The maximum likelihood tree showed that the isolates are distributed in three main groups with almost 100% similarity within each one, with only the CBSAL09 strain as an exception (Figure 1).
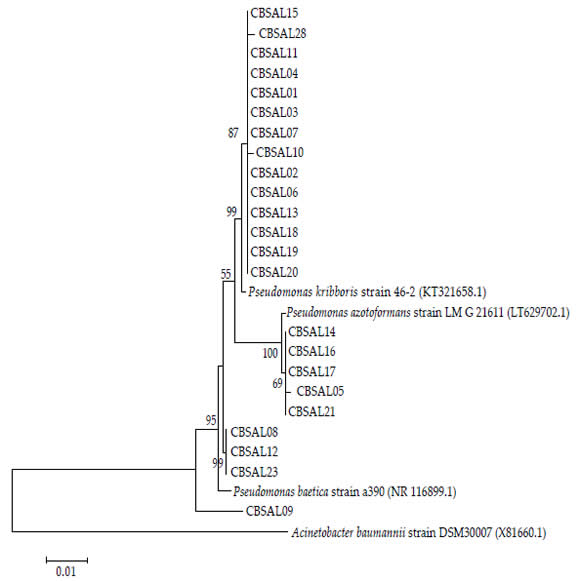
Figure 1. Neighbor Joining tree based on 16S rRNA, (2571 bp) showing the phylogenetic relationship between Pseudomonas isolates
from this work and reference strains (GenBank). Support values (1000 bootstrap replicates >50%) are shown. Scale bar in number of
substitutions per site.
Figura 1. Árbol de unión vecina basado en 16S rRNA, (2571 pb) que muestra la relación filogenética entre los aislados de
Pseudomonas de este trabajo y las cepas de referencia (GenBank). Se muestran los valores de soporte (1000 réplicas de arranque>
50%). Barra de escala en número de sustituciones por sitio.
EVALUATION OF PLANT GROWTH-INDUCING
In vitro ANALYSIS
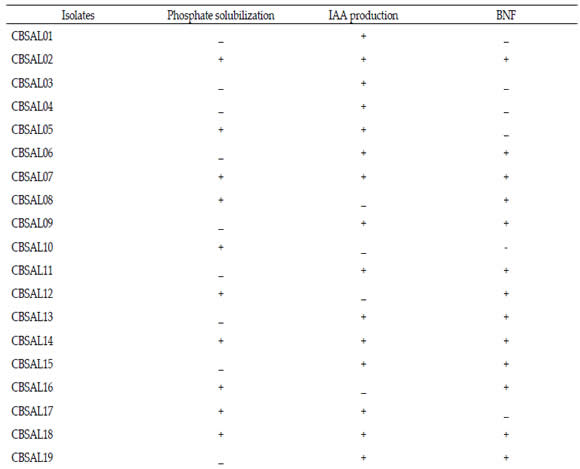
The PGPRs promotion of plant growth, as fluorescent Pseudomonas, involves direct mechanisms, such as phosphates solubilization (Cattelan, 1999) and indirect ones, as phytopathogens suppression (Botelho & Mendonça- Hagler, 2006). Phosphate solubilization, IAA production and biological N fixation were the direct induction mechanisms investigated to the isolates obtained from garlic (Table 3). It was observed that more than half of the isolates showed solubilization halo around the colony, characterizing positive reaction. From 23 isolates, twelve (52,2%) were able to solubilize calcium phosphate. Regarding the production of IAA, there were eighteen bacteria (78,0%) that had positive reaction and sixteen isolates (69,6%) formed pellicle in NFB medium, indicating ability to fix N in vitro. It was observed that seven garlic isolates (30,40%) had two of the induction mechanisms evaluated while five (21,70%) had the three.
Table 3. Isolates plant growth promotion mechanisms in vitro.
Tabla 3. Mecanismos de promoción de crecimiento de plantas de los aislados in vitro.

ANALYSIS in planta
Based on the phosphate solubilization and IAA production, four isolates that showed positive reaction (CBSAL02, CBSAL05, CBSAL14, CBSAL18) were selected to evaluate their potential of inducing plant development. There was no significant difference to root dry weight means (Figure 2). Treatments effect was significant for the parameters plant height, fourth leaf size, dry and wet shoot weight and wet weight root (Figure 2). For plant height, dry and wet shoot weight, isolates were in the same statistical group of control, although the CBSAL02 had higher mean values than that one (differences of 7,4%, 27,6% and 21,7%, respectively). For fourth leaf size, CBSAL02 was significantly higher than control (difference of 27,6%). For the wet weight root, isolate CBSAL14 was significantly lower than the control (difference of 47,3%). Isolates CBSAL02 e CBSAL14 were different from each other to plant height, wet and dry shoot weight at differences of 15,8%, 51,4% and 43,5%, respectively.
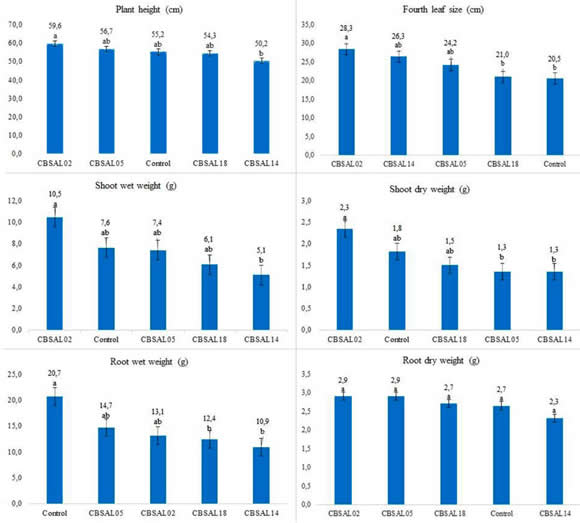
The numbers on the bars represent treatment averages compared by Tukey's test at 5% of significance. Treatments followed by the same letter
are not significantly different.
Figure 2. Fluorescent Pseudomonas inoculation effect on garlic grown in greenhouse 110 days after sowing.
Figura 2. Efecto de inoculación de Pseudomonas fluorescente ajo cultivado en casa de vegetación 110 días después de la siembra.
DISCUSSION
Although the phenotypic analyzes, especially the morphological ones are not definitive for identifying a bacterium, these can significantly assist in the characterization of diverse bacterial groups such as Pseudomonas. The green fluorescence and the bright intensity observed at major part of isolates on King B medium seems to a common morphological characteristic of fluorescent Pseudomonas. Ferreira et al. (2009) observed similar results, in which few isolates had low fluorescence intensity. On the other hand, the shape and edge, are not described as significant for fluorescent Pseudomonas differentiation. However, most species of this genus have morphological feature of colony smooth edge and circular shape (Fonseca et al., 2000). This was observed for most of the isolates of garlic.
Most of the isolates showed convex elevation. According to Fonseca et al. (2000), it is common to the genus Pseudomonas and this characteristic is related, in most cases, to the presence of mucus. According to Zago et al. (2000), to assist in the differentiation of Pseudomonas species, using the morphology of colonies, the elasticity of the mucus is significantly relevant. Lower elasticity was observed in colonies of P. putida compared the P. fluorescens.
The biochemical evaluation showed characteristic results of fluorescent Pseudomonas, for most of the isolates (Table 2). All isolates were negative for fermentation of glucose which is a common characteristic of the species P. putida and P. fluorescens, species typically found in the rhizosphere. They use other routes of glucose utilization, like Entner-Doudoroff pathway (Ribeiro & Soares, 2002). This observation could be confirmed by the Vogues Proskauer test that verifies the production of acetoin, from the fermentation of glucose, through the butylene glycol pathway. Pseudomonas present negative reaction when submitted to the test.
Most isolates were able to degrade urea. Despite the great variability observed among isolates of different habitats, this characteristic can aid to the species differentiation, since it was observed that among isolates of species, such as P. fluorescens and P. aeruginosa it was present and to others, like P. putida and P. stutzeri , absent (Abdelzaher et al., 2004; Mehnaz & Lazarovits, 2006). Among the tests, it was one of them that had the most positive responses. This suggested adaptability to the conditions, since garlic is a demanding plant in N (Lucini, 2009) and currently, urea is the main source of N in Brazilian agriculture, and cultivars are quite responsive to this.
Catalase presence was observed for most isolates and like urease test, most isolate was positive to it. The presence of catalase is common in the species of Pseudomonas (Holt et al., 1994), as well as the absence of growth at 41°C (Showkat et al., 2012), indicating that these two parameters can help at phenotypic characterization.
The biochemical characterization is important in detecting the activity of microorganisms and can be a useful tool in the identification of these, especially for few isolates like this. However, for a greater number of individuals, due to the metabolic diversity, especially of complex bacterial groups, such as the genus Pseudomonas, it can lead to inconclusive results, even when using microbial identification kits. In addition, it may underestimate diversity-related data in microbial ecology studies, by assessing only functional diversity (Coelho, 2006). For this reason, genotypic characterization became a relevant procedure for the identification and analysis of microorganisms in the environment (Mehnaz & Lazarovits, 2006; Kim et al., 2013).
The 23 bacteria isolates obtained from garlic rhizosphere show similarity above 99% with strains from genus Pseudomonas, confirming that all belong to this genus. The analyzes, Blast seach and phylogenetics, showed that the isolates are distributed within three groups (Figure 1) and each group has the type strains of Pseudomonas kribbensis, P. azotoformans or P. baetica as the closest ones. All these three species, as well as other related species, belong to Pseudomonas fluorescens-related species group (Anzai et al., 2000), which are commonly bacteria found in the environment. Currently, the phylogenetic analysis of 16S rRNA is a marker that indicates which genus a given bacteria belongs, not confirming the exact species. In this way, further analysis will be necessary for the confirmation of the species to which these bacteria belong.
The growth induction mechanisms tested are widely described to several PGPRs, at different plant species (Gage, 2004; Karnwal, 2009; Bashan & Bashan, 2010; Ayyaz et al., 2016). It is important that these mechanisms are properly described to PGPRs, especially those, such as fluorescent Pseudomonas which have potential for use in sustainable agricultural systems.
The ability to solubilize phosphates by microorganisms becomes important for plant development, since much of the phosphorus in the soil is unavailable because it is bound to organic and inorganic components (Grant et al., 2001; Cerezini et al., 2009). At this work, more than half of the isolates (52,17%) had phosphate solubilization capacity. It was a significant amount when compared to the results of phosphate solubilizing rhizobacteria obtained by other authors (Coelho, 2006; Pedrinho et al., 2010; Ambrosini et al., 2012). Ambrozini et al. (2012) evaluated the diversity of sunflower-associated rhizobacteria and found 59 phosphate solubilizing isolates in a total of 299 (around 20%). This result was similar to that found by Coelho (2006) to different plants. Pedrinho et al. (2010) found that about 46% of maize isolates showed solubilizing halo on the medium, which varied along the incubation period. Our results became especially important since all isolates were fluorescent Pseudomonas that are present at large communities in the crops rhizosphere (Botelho & Mendonça-Hagler, 2006). They are described as efficient phosphate solubilizers (Park et al., 2009; Yadav et al., 2016) and able of increasing crop development when associated to phosphate fertilizers (Chaves et al., 2013).
The existence of a phosphate solubilizing community associated to the garlic roots can decrease the amount of phosphorus fertilization, aiding the absorption of the nutrient by the plants and therefore, increasing productivity and reducing environmental effects. The garlic demands for P is lower when compared to N and K. However, its deficiency may cause reduction in plant growth and at the bulbils amount (Macêdo et al., 2011).
Regarding the production of IAA, it was observed that this mechanism was the most significant among the isolates. Seventy-eight percent of the collection showed a positive reaction. This agrees with several reports indicating the IAA production is a common mechanism among PGPRs, including Pseudomonas genus (Karnwal, 2009; Bashan & Bashan, 2010; Meliani et al., 2017). The percentage of IAA producers was significant when compared to the percentages among PGPRs producers that are around 50% (Pedrinho et al., 2010; Cipriano et al., 2013) and among fluorescent Pseudomonas community that reached 45 to 67% (Wahyudi; Astuti & Giyanto, 2011). The occurrence of large IAA producer community associated to the garlic roots can induce their growth, stimulating the nutrients absorption, especially N, which garlic is demanding, increasing its productivity and reducing fertilization.
BNF was also significant among isolates, although it was not used as the main parameter for the selection, for the in vivo test. Sixteen isolates (69,60%) showed pellicle formation at NFB medium. The results are in accord to several authors that described the ability to fix N for the genus Pseudomonas (Watanabe et al., 1987; Fernandes et al., 2001; Mirza et al., 2006; Li et al., 2017), especially for the species P. stutzeri (Desnoues et al., 2003; Venieraki et al., 2014), and even new species (Watanabe et al., 1987; Venieraki et al., 2014). Fernandes et al. (2001) reported that among the isolates of diazotrophic bacteria from coconut palms, two identified as Pseudomonas sp. had the highest potential of growth and high BNF capacity.
In our study, four isolates (CBSAL02, CBSAL05, CBSAL14 and CBSAL18) were selected for evaluation of their ability to stimulate plant growth, based on their phosphate solubilization capacity and IAA production. Among them, only CBSAL05 did not have positive reaction to BNF.
EVALUATION OF PLANT GROWTH-INDUCING
In planta ANALYSIS
To the parameters checked, there was no statistical difference comparing the isolates to the control, except to fourth leaf size and root wet weight. However, CBSAL02 had the highest average for all parameters tested, except for root dry weight. Possibly, the differences were not attested statistically, at the end of the experiment, due to little space for root expansion, resulting in plant growth stabilization, underestimating the inoculation effect observed at the beginning of development, when it was more evident. Goswami et al. (2013) observed that chickpea stimulation and mung growth by fluorescent Pseudomonas already occurred in the early stages of development. The inoculated seedlings showed a significant increase in dry and wet mass after 10 to 15 days after sowing.
By the evaluation of the fourth leaf size, the isolate CBSAL02 was significantly higher than the control. In the stage of bulb differentiation, the measurement of the fourth leaf can be an indicative of the bulb production, due to the high correlation between the parameters at the field analyzes (Biasi,1986). This suggested that the CBSAL02 can increase the development and production of garlic.
The CBSAL14 isolate had the lowest mean values to the parameters checked, except to fourth leaf size, which may suggest little or no correlation to induction mechanisms when in contact to the plant. It is known that in soil, plant genotype modulates the microbial rhizosphere community size and diversity (Berg & Smalla, 2009; Schreiter et al., 2018) that could prevent the development of microorganisms that compete excessively for nutrients with the plant. The absence of this community at the sterilized substrate could explain the negative effect of CBSAL14 on plant development. Possibly, it had high multiplication capacity and/or nutrient competition ability that reflected on plant growth parameters assessed.
Our results are supported by several authors observations that indicated the PGPR, including fluorescent Pseudomonas, inoculation stimulates plant development, especially those which have mechanisms such as phosphate solubilization and IAA production (Mercado-Blanco & Bakker, 2007; Lucon et al., 2008; Karnwal, 2009; Pedrinho et al., 2010). Goswami et al.(2013) observed that isolates positives to phosphate solubilization, IAA production and other mechanisms promoted the growth of chickpea and mung. Chattoo et al. (2007) described the efficiency of rhizobacteria associated with lower fertilizer dosages at garlic, suggesting that these were able to assist in plant nutrition. It is important to highlight that many of these described PGPR are fluorescent Pseudomonas.
There are few studies of rhizobacteria effect on garlic, especially to direct growth induction. These results indicated that major part of fluorescent Pseudomonas isolates from garlic solubilized phosphate and produced IAA in vitro, suggesting potential as biofertilizers, as observed to CBSAL02 isolate that significantly induced the fourth leaf development in greenhouse and will be evaluated at field conditions.
The 16S rRNA sequencing showed that all isolates belonged to genus Pseudomonas and were closest related to the species Pseudomonas kribbensis, P. azotoformans or P. baetica.
ACKNOWLEDGMENT
Agronomist Engineer Roberto de Almeida, for the agricultural inputs donation and for the constant support.
A Cooperalho de Curitibanos (SC) for the seed donation.
Mr. André Gustavo de Luca Dias, owner of the Dias farm, in the locality of Horizolândia, Curitibanos (SC), for the soil that made possible the beginning of this work.
The former EPAGRI (SC) agronomists M.A.Lucini and J. Biasi, for valuable information about the garlic crop.
Research Group in Microrganismos Promotores de Crescimento de Plantas (MPCP) (Plant Growth Promoters Microorganisms) from UFSC campus de Curitibanos. email: mpcp-cbs@mailman.ufsc.br.
REFERENCES
1. Agaras, BC; M Scandiani; A Luque; L Fernández; F Farina;M Carmona; M Gally; A Romero; L Wall & C Valverde. 2015. Quantification of the potential biocontrol and direct plant growth promotion abilities based on multiple biological traits distinguish different groups of Pseudomonas spp. isolates. Biol. Control 90: 173-186. Doi: 10.1016/j.biocontrol. 2015.07.003.
2. Abdelzaher, HMA; MM Imam; MA Shoulkamy & YMA Gherbawy. 2004. Biological control of Pythium damping-off of bush okra using rhizosphere strains of Pseudomonas fluorescens. Mycobiol. 32 (3): 139-147.
3. Ambrosini, A; A Beneduzi; T Stefanski; FG Pinheiro; LK Vargas & LMP Passaglia. 2012. Screening of plant growth-promoting rhizobacteria isolated from sunflower (Helianthus annuus l.). Plant Soil 356: 245-246. Doi: 10.1007/s11104-011-1079-1.
4. Anzai, Y; H Kim; JY Park; H Wakabayashi & H Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int J Syst Evol Microbiol. 50 (4):1563-89. Doi: 10.1099/00207713-50-4-1563.
5. Ayyaz, K; A Zaheer; G Rasul & MS Mirza. 2016. Isolation and identification by 16S rRNA sequence analysis of plant growth-promoting azospirilla from the rhizosphere of wheat. Braz J Microbiol 47(2): 542-550. Doi: 10.1016/j.bjm.2015.11.035.
6. Bashan, Y & LE Bashan. 2010. How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108: 77 - 136. Doi: 10.1016/S0065-2113(10)08002-8.
7. Berg, G & K Smalla. 2009. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68: 1-13. Doi: 10.1111/j.1574-6941.2009.00654.x
8. Biasi, J. 1986. Previsão da produção por ocasião da diferenciação dos bulbilhos de alho. Hortic Bras 4(1): 47.
9. Biasi, J. 2006. Nutrição e indicação de adubação para a cultura do alho, Boletim técnico, 132. Epagri. [Florianópolis].
10. Botelho, GR & LC Mendonça-Hagler. 2006. Fluorescent pseudomonads associated with the rhizosphere of crops - an overview. Braz J Microbiol 37: 401-416. Doi: 10.1590/S1517-83822006000400001.
11. Botelho, GR; MCC Fonseca; MCP Neves; GR Xavier & NG Rumjanek. 2011. Profile of fluorescent pseudomonads for biocontrol use isolated from a Brazilian agroecological production system. UNICiên. 15(1): 333-354.
12. Cattelan, AJ. 1999. Métodos quantitativos para determinação de características bioquímicas e fisiológicas associadas com bactérias promotoras do crescimento vegetal, Documentos 139. Embrapa soja. [Londrina].
13. Cerezini, P; KLM Milani & EL Balota. 2009. Seleção de microrganismos solubilizadores de fosfato. Synerg. Scyent. 4 (1). http://revistas.utfpr.edu.br/pb/index.php/SysScy/ article/viewFile/597/343. 16 january 2019.
14. Chattoo, MA; N Ahmed; S Faheema; S Narayan; SH Khan & K Hussain. 2007. Response of garlic (Allium sativum L.) to biofertilizer application. The Asian J Hortic. 2 (2): 249-252.
15. Chaves, DP; C Zucareli & A de Oliveira Junior. 2013. Fontes de fósforo associadas à inoculação com Pseudomonas fluorescens no desenvolvimento e produtividade do milho. Semina: Ciências Agrárias 34 (1): 57-72. DOI: 10.5433/1679-0359.2013v34n1p57.
16. Cipriano, MAP; FRA Patrício & S Dos S Freitas. 2013. Potencial de rizobactérias na promoção de crescimento e controle da podridão radicular em alface hidropônica. Summa Phytopathol.39 (1): 51-57.
17. Companhia Nacional De Abastecimento (CONAB). Conjuntura mensal: alho. Dezembro/2017. 2017. file:///C:/Users/ Gl%C3%B3ria/Downloads/Alho_-_Analise_Mensal_-_ dezembro-2017.pdf. 04 de may 2018.
18. Coelho, LF. 2006. Interação de Pseudomonas spp. e de Bacillus spp.com diferentes rizosferas. Dissertação de mestrado, Instituto Agronômico. [Campinas, Brasil].
19. Desnoues, N; M Lin; X Guo; L Ma; R Carreño-Lopez & C Elmerich. 2003. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiol. 149: 2251-2262. Doi: 10.1099/ mic.0.26270-0.
20. Döbereiner, J; VL Baldani & JI Baldani. 1995. Como isolar e identificar bactérias diazotróficas de plantas não-leguminosas. Embrapa - Centro Nacional de Pesquisa em Agrobiologia. [Seropédica, RJ, Brasil].
21. Dos Santos HG et al. 2013. Sistema Brasileiro de Classificação de Solos. 3 ed. rev. ampl. Embrapa. [Brasília, DF, Brasil].
22. Drogue, B; H Doré; S Borland; F Wisniewski-Dyé & C Prigent- Combaret. 2012. Which specifity in cooperation between phytostimulating rhizobacteria and plants? Res Microbiol 13: 500-510. Doi: 10.1016/j.resmic. 2012.08.006.
23. Fernandes, MF; RPM Fernandes & L Da S Rodrigues. 2001. Bactérias diazotróficas associadas a coqueiros na região de baixada litorânea em Sergipe. Pesq. agropec. bras. 36 (12): 1509-1517.
24. Ferreira, EPB; M Voss; HP Santos; H PollI; MCP Neves & NG Rumjanek. 2009. Diversidade de Pseudomonas fluorescentes em diferentes sistemas de manejo do solo e rotação de culturas. Rev. Bras. Ciênc. Agrária 4(2): 140- 148. Doi: 10.5039/agraria.v4i2a4.
25. Fonseca, MCC; VCP Zago; EPB Ferreira; AFS Câmara & NG Rumjanek. 2000. Isolamento e caracterização morfológica de Pseudomonas spp. fluorescentes nativas em sistemas de produção agrícola (Comunicado Técnico). Embrapa Agrobiologia 43: 1-4. http://www.infoteca.cnptia. embrapa.br/bitstream/doc/623981/1/cot043.pdf . 30 august 2014.
26. Freitas, SS & CI Aguilar-Vildoso. 2004. Rizobactérias e promoção de crescimento de plantas cítricas. Rev Bras Cienc. Solo 28: 987-984.
27. Gage, DJ. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol R 68(2): 280-300. Doi: 10.1128/ MMBR.68.2.280-300.2004.
28. Goswami, D; H Vaghela; S Parmar; P Dhandhukia & JN Thakker. 2013. Plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. J Plant Interact 8 (4): 281-290. Doi: 10.1080/17429145.2013.768360.
29. Gouda, S; RG Kerry; G Das; S Paramithiotis; HS Shine & JK Patra. 2018. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microb Res 206: 131-140. Doi: 10.1016/j. micres.2017.08.016.
30. Grant, CA; DN Flaten; DJ Tomasiewicz & SC Sheppard. 2001 A importância do fósforo no desenvolvimento inicial da planta. Informações Agronômicas 95. Potafos. [Piracicaba, Brasil].
31. Hoagland, D & DI Arnon. 1950. The water culture method for growing plants without soil.California Agricultural Experiment Station Circular 347. College of Agriculture, University of California. [Berkeley, EUA].
32. Holt, JG; NR Krieg; PHA Sneath; JT Staley & ST Williams. 1994. Bergey's Manual of Determinative Bacteriology. Nineth ed. Williams & Wilkins. [Baltimore, EUA].
33. Hungria, M & IC Mendes. 2015. Nitrogen fixation with soybean: the perfect symbiosis? Em: FJ De Bruijn (ed.). Biological Nitrogen Fixation. Wiley. [Hoboken, EUA].
34. Karnwal, A. 2009. Production of indole acetic acid by fluorescent Pseudomonas in the presence of L-tryptophan and rice root exudates. J Plant Pathol 91(1): 61-63.
35. Kim, JS; PM Mele & DE Crowley. 2013. Application of PCR primer sets for detection of Pseudomonas sp. functional genes in the plant rhizosphere. J. Agric. Chem. Env. 2: 8-15.
36. King, EO; MK Ward MK & DE Raney.1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301-307.
37. Li, HB; RK Singh; P Singh; Q-Q Song; Y-X Xing; L-T Yang & Y-R Li. 2017. Genetic diversity of nitrogen-fixing and plant growth promoting Pseudomonas species isolated from sugarcane rhizosphere. Front Microbiol 8: 1 - 20. Doi: 10.3389/fmicb.2017.01268.
38. Loper, JE & JS Buyer. 1991. Siderophores in microbial interactions on plant surfaces. Mol Plant Microbe In 4: 5-13. Doi: 10.1094/MPMI-4-005.
39. Lucini, MA. 2009. Principais doenças fúngicas na cultura do alho. 2nd ed. EPAGRI. [Curitibanos, Brasil].
40. Lucon, CMM; MA Akamatsu & R Harakava. 2008. Promoção de crescimento e controle de tombamento de plântulas de pepino por rizobactérias. Pesq. agropec. bras. 43 (6): 691-697.
41. Macêdo, FS; ET Sedoguchi; RJ de Souza RJ & JG de Carvalho. 2011. Produtividade de alho vernalizado em função de fontes e doses de fósforo. Cienc Rural 41(3): 379- 383.
42. Meliani, A; A Bensoltane; L Benidire & K Oufdou K. 2017. Plant growth-promotion and iaa secretion with Pseudomonas fluorescens and Pseudomonas putida. Res Rev: J Bot Sci 6 (2): 16-24.
43. Melo, LS & PJ Valadrini. 1995. Potencial de rizobactérias no controle de Fusarium solani (Mart.) Sacc. em pepino (Cucumis sativum L.). Sci Agr 52(2): 326-330. Doi: 10.1590/S0103-90161995000200020.
44. Mehnaz, S & G Lazarovits. 2006. Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb Ecol 51: 326-335. Doi: 10.1007/s00248-006-9039-7.
45. Mercado-Blanco, J & PAHM Bakker. 2007. Interactions between plants and beneficial Pseudomonas spp.: exploiting bacterial traits for crop protection. A Van Leeuw J Microb 92: 367-389. Doi: 10.1007/s10482-007- 9167-1.
46. Mirza, MS; S Mehnaz; P Normand; C Prigent-Combaret; Y Moënne-Loccoz ; R Bally & KA, Malik. 2006. Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonas strain promoting rice growth. Biol Fertil Soil 43: 163-170. Doi: 10.1007/s00374-006-0074-9.
47. Moreira, FM de S; K da Silva; RS Abrahão & F de C Nóbrega. 2010. Bactérias diazotróficas associativas: diversidade, ecologia e potencial de aplicações. Comun. Sci 1(2): 74- 99.
48. Novakowiski, JH; IE Sandini; MK Falbo; A Moraes A; JH Novakowiski & NC Cheng. 2011. Efeito residual da adubação nitrogenada e inoculação de Azospirillum brasilense na cultura do milho. Semina-Cienc Agrar 32 (1): 1687-1698.
49. Ogata‑Gutierrez, K; C Chumpitaz‑Segovia; J Lirio‑Paredes; MM Finetti‑Sialer & D Zuniga‑Davila. 2017. Characterization and potential of plant growth promoting rhizobacteria isolated from native Andean crops. World J Microb Biot 33: 203-216. doi: 10.1007/s11274-017-2369-4.
50. Park, KH; CY Lee & HJ Son. 2009. Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Letters in Applied Microbiology 49: 222-228. Doi: 10.1111/j.1472- 765X.2009.02642.x.
51. Pedrinho, EAN; RF Galdiano Júnior; JC Campanharo; LMC Alves & EGM Lemos. 2010. Identificação e avaliação de rizobactérias isoladas de raízes de milho. Bragantia 69 (4): 905-911.
52. Pires, RdeC; F.B. dos Reis Júnior; JE Zilli; D Fisher; A Hoffmann; EK James; MF Simon. 2018. Soil characteristics determine the rhizobia in association with different species of Mimosa in central Brazil. Plant Soil 423: 411-428.
53. Raaijmakers, JM & M Mazzola. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 50: 403- 24. Doi: 10.1146/annurev-phyto-081211-172908.
54. Ribeiro, MC & MMSR Soares. 2002. Microbiologia Prática: Roteiro e Manual. Bactérias e Fungo. Atheneu. [São Paulo, Brasil].
55. Schreiter, S; D Babin; K Smalla & R Grosch. 2018. Rhizosphere competence and biocontrol effect of Pseudomonas sp. RU47 independent from plant species and soil type at the field scale. Frontiers im Microbiology 9 (97):1-10. Doi: 10.3389/fmicb.2018.00097
56. Sivasakthi, S; G Usharani & P Saranraj. 2014. Biological potentiality of plant growth promoting bactéria (PGPR) - Pseudomonas fluorescens and Bacillus subtilis: a review. Afr J Agric Res 9(16): 1265-1277. Doi: 10.5897/ AJAR2013.7914.
57. Showkat, S; I Murtaza; O Laila & A Ali. 2012. Biological control of Fusarium oxysporum and Aspergillus by Pseudomonas fluorescens isolated from wheat rhizosphere soil of Kashmir. J Pharm Pharm Sci 1: 24-32. Doi: 10.9790/3008-0142432.
58. Souza, RJ & FS Macedo. 2009. Cultura do alho: tecnologias modernas de produção. Ed Editora UFLA. [Lavras, Brasil].
59. Turatto, MF; F dos S Dourado; JE Zilli & GR Botelho. 2018. Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz J Microbiol 49: 54-58. Doi: 10.1016/j. bjm.2017.03.015.
60. Watanabe, I; SJ Rolando; K Ladha; Y Katayama-Fujimura & H Kuraishi.1987. A new nitrogen-fixing species of pseudomonad: Pseudomonas diazotrophicus sp. nov. isolated from the root of wetland rice. Can J Microbiol 33 (8): 670-678.
61. Wolska, K & P Szweda. 2012. Genotyping techniques for determining the diversity of microorganisms. Em: M Caliskan (ed.). Genetic diversity in microorganisms. In Tech. [Croatia, pp 53-94].
62. Venieraki, A; M Dimou; E Vezyri; A Vamvakas; P-A Katinaki; I Chatzipavlidis; A Tampakaki & P Katinakis. 2014. The nitrogen-fixation island insertion site is conserved in diazotrophic Pseudomonas stutzeri and Pseudomonas sp. isolated from distal and close geographical regions. PLOS ONE 9 (9): 1-8. Doi: 10.1371/journal.pone.0105837.
63. Voisard, C; CT Bull; C Keel & J Laville.1994. Biocontrol of root disease by Pseudomonas fluorescens CHA0: current concepts and experimental approaches. In: O'gara, F; DN Dowling & B Boesten (eds). Molecular ecology of rhizosphere microorganisms. VCH. [Germany, pp. 67-89].
64. Wahyudi, AT; RI Astuti & G Giyanto. 2011. Screening of Pseudomonas sp. isolated from rhizosphere of soybean plant as plant growth promoter and biocontrol agent. American Journal of Agricultural and Biological Sciences 6 (1): 134-141.
65. Yadav, A; K Yadav & A Vashistha. 2016. Phosphate solubilizing activity of Pseudomonas fluorescens PSM1 isolated from wheat rhizosphere. Journal of Applied and Natural Science 8 (1): 93 - 96.
66. Yu, JM; D Wang; LS Pierson III & EA Pierson. 2018. Effect of producing different phenazines on bacterial fitness and biological control in Pseudomonas chlororaphis 30-84. Plant Pathology J 34(1): 44-58. Doi: 10.5423/PPJ. FT.12.2017.0277.
67. Zago, V; H De-Polli; N Rumjanek. Pseudomonas spp. fluorescentes - Bactérias promotoras de crescimento de plantas e biocontroladoras de fitopatógenos em sistemas de produção agrícola. Seropédica: EMBRAPA Agrobiología/EMBRAPA- CNPAB, 2000. 32p. (Documento, 127)